Provide consumers with confidence that your product is safe to use, while building brand recognition as a high-quality product in the marketplace.
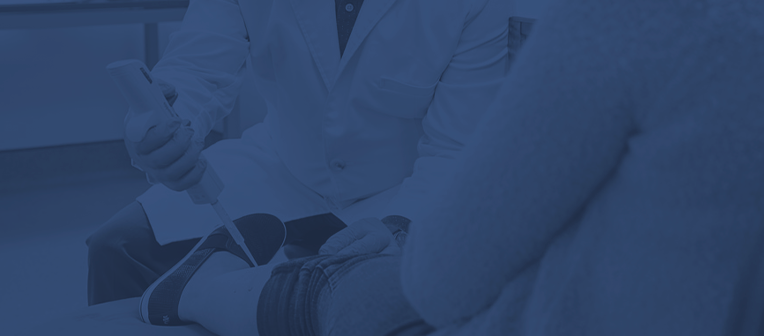
Irritation Testing
- Acute Irritation (24-hour/ 48-hour patch local tolerance test to assess Primary Irritation)
- Cumulative Irritation (7-/14- and 21-Days)
- Comedogenicity
- Soap (Mildness) Testing
Testing that is customizable yet based on established industry procedures in product research and development! Substantiate your label claims, ensuring your packaging and advertisements are compliant with regulatory requirements.
Photosensitization and phototoxicity Testing
Prove that your ingredients are safe for use in an in vivo situation when the product is exposed to UV light and allows you to make safety claims.
Claim Substantiation for: Non-Phototoxic, Non-Photosensitive
UVA, UVB and SPF testing for sunscreen products
SPF & UVB testing: Examine your ingredients within the regulatory measures for SPF in an in vivo situation allowing you to make an SPF claim on the packaging and in advertisements!
- Methods: COLIPA Method / ISO: 24444 Method – International, FDA Approved Method – USA / Canada, or Australian / New Zealand Approved Method
- Claim: SPF Rating
UVA testing: Examine your ingredients within the regulatory measures for UVA in an in vivo situation allowing you to make an UVA claim on the packaging and in advertisements!
-
-
- Method: COLIPA Method / ISO: 24443 Method – International
- Claim: UVA protection
-
Wound Care Products
Tests devices and various topical products used in would care such as: fabrics, films, foams, adhesives, ointments, active ingredients / bioactives, and more.
Evaluations: Adhesiveness, Erythema, Exudate absorption, Pain ,Skin Barrier protection, Skin Barrier Recovery, Skin damage with removal , Skin and wound healing and more.
Content missing

Content missing

Content missing

Content missing

Content missing
